By Richard McLoughlin
Bringing a new medical device product to market is an intensive and extensive process, so it’s important you get the right support. The best approach is to work with a partner that offers a complete service offering, with end-to-end support from the concept development stage through to full-scale manufacturing. At Arrotek, we offer this full range of services.
Arrotek is a well-established contract manufacturer of custom needles, stylets, cannulas, and similar medical device components. In addition, our design and development capabilities extend to guidewires and specialist catheter technologies. We can support your project wherever you are within the development journey.
Furthermore, we have expertise supporting all types of customers in the medical device industry, from early-stage start-ups to the largest multinationals.
Design Services – Our Six-Step Medical Device Design Process
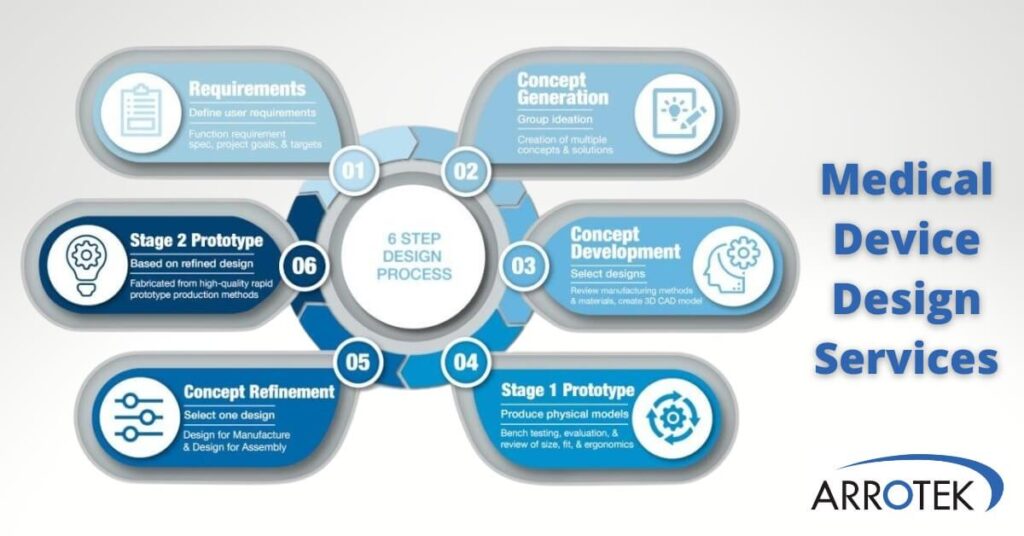
We use a robust, tried-and-tested six-step process when both designing new medical device products and updating existing products.

The six steps include:
- Step 1 – Defining user requirements, the functional specification, and the project’s goals and targets.
- Step 2 – Concept generation where our team develops multiple ideas and potential solutions.
- Step 3 – Concept development where the selected design is further improved with the creation of 3D CAD models. Materials and manufacturing methods are also evaluated in this step.
- Step 4 – Production of a Stage 1 Prototype for bench testing and practical evaluation.
- Step 5 – Product refinement based on the results of the prototype evaluation, with additional DFM and DFA considerations (see below).
- Step 6 – Production of quality-controlled Stage 2 Prototypes of your product for further testing and trials.
Complete Service Offering – Design, Prototyping, Regulatory, and Manufacturing
We also provide expert support once your product moves out of the design stage. This includes regulatory and manufacturing support:

- Documentation support, including the design history file, as well as manufacturing, quality, risk, and validation documentation
- Packaging development
- Development and validation of sterilization processes
- Biocompatibility testing
- Design and fabrication of the tooling required to manufacture your product
- The manufacture of product batches for design and process verification
- Full-scale manufacturing
- Assembly and packaging
Benefits of Arrotek’s Design Services
While we provide the design services and additional support you need to bring your medical device product to market, it is our approach that sets us apart. The benefits of Arrotek’s design services include:
Bandwidth
Working with our sister company Arrotek, we have the bandwidth available to meet your medical device design requirements. This will help keep your project timelines on track and avoid any unnecessary delays.
Modular Approach
We use a modular approach in the delivery of our design services at Arrotek. This means we can support your project from start to finish, including taking on a project management role. Our modular approach means we can also provide support on a specific element of a larger project.
In other words, whatever you need, we’ll tailor the services that we provide.
Time and Materials Option
Our time and materials offering is available to all clients, but it is particularly favored by smaller businesses and start-ups that are not yet ready to commit to large, resource-intensive, and expensive contracts.
With our time and materials offering, you can purchase the design services you need, when you need them. This helps to control costs and it ensures the development process is fully adapted to your requirements.
DFM and DFA
DFM (Design for Manufacturing) and DFA (Design for Assembly) are crucial considerations for our engineers during the design process. Both DFM and DFA involve ensuring your product can be efficiently and cost-effectively produced.
As we are a medical device contract manufacturer, we bring a wealth of experience to the DFM and DFA elements of the design process.
Prototyping Capabilities
The production of prototypes is essential in the medical device design and development process. At Arrotek, we have rapid prototyping capabilities that enable us to produce prototypes for a range of purposes. This includes concept evaluation and refinement prototypes, as well as quality-controlled prototypes for clinical evaluation.
Expertise and Experience
We’ll bring wide-ranging experience and expertise to your medical device project:
- Needle, catheter, and minimally invasive medical device expertise
- Material expertise, including stainless steel and polymers
- Experience developing cutting-edge medical device solutions utilizing the latest technologies while pushing the boundaries of material and component limitations
- Experience working with start-up companies, international medical device corporations, and everything in between
- Expertise in advanced production techniques
- Regulatory expertise and practical support
Find Out More About Our Design Services
Whatever stage you have reached in your product development journey, we’d love to discuss how Arrotek’s design services division can provide the support you need. Get in touch by completing the form below and a member of our team will get back to you.